Medical network February 28th hearing
Cardiovascular drug use fell slightly
But the steady growth of lipid-lowering drugs
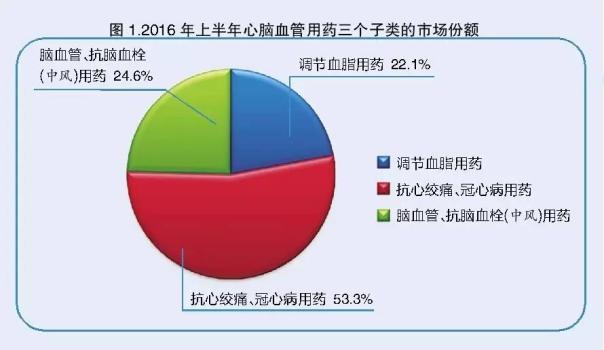
In the domestic and international economic situation is complex and changeable and competitive market environment, the pharmaceutical retail industry growth is from rapid growth to steady growth. As one of the main categories of drugs in the retail market of drugs slow disease, cardiovascular drugs are no exception, the first half of 2016, 22 cities total sales growth of 6%, down by 0.3%. Anti angina, coronary heart disease drugs are cardiovascular drugs in the largest subclass of stable market share accounted for half of the country, followed by cerebrovascular and cerebral thrombosis (stroke) medication, the market share of nearly 1/4, while the lipid medication market share of 22.1% (see Figure 1).
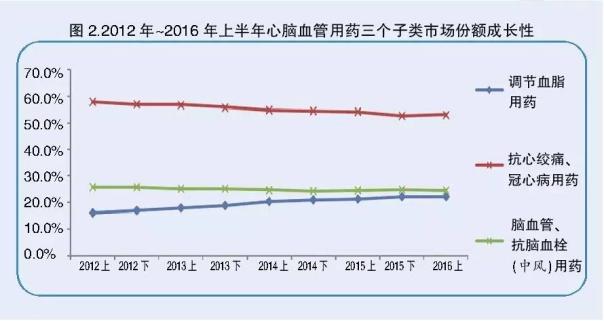
 Although the drug lipid regulating market share ranked on three after the drug has maintained a large growth in the first half of 2016, an increase of 3.9%, 2012 to the first half of 2016 semi annual compound growth rate of 11%, the market share in the regulation of blood lipid drug gradually increased (see Figure 2), the market expansion speed fast, considerable incremental market, a good imagination space for future market.
 Cardiovascular and cerebrovascular diseases for chronic diseases, treatment of traditional Chinese medicine has unique advantages in this aspect, the 22 city total cardiovascular medicine in traditional Chinese medicine, with a market share of 70% way ahead, which is mainly composed of cardiovascular drugs in a larger proportion of angina, coronary heart disease medication and cerebral blood vessels and cerebral thrombosis (stroke) medication two sub class contribution caused by cardiovascular drugs in the fastest-growing drug is to regulate blood lipid as the leading chemical drugs.
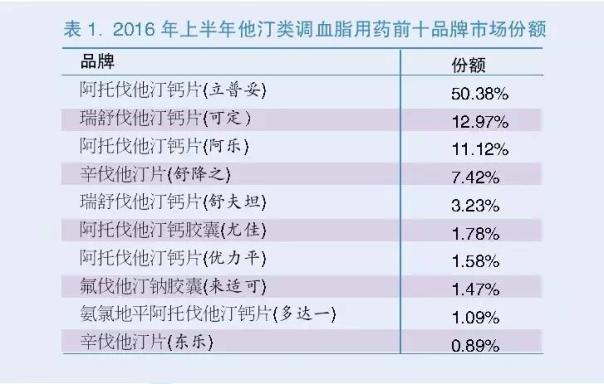
According to the therapeutic effect of different medication, blood lipid regulation is mainly divided into: to reduce blood total cholesterol and low density lipoprotein - such as statins, atorvastatin, simvastatin and pravastatin; to reduce triglyceride based fibrates, such as fenofibrate and bezafibrate; and others, such as acid, polyunsaturated fatty acid other drugs. Among them, statins with more than 90% market share, and share gradually increased (see Figure 3).
Statins and HMG formyl coenzyme A (HMG-CoA) is similar to that of HMG-CoA can inhibit the transformation to mevalonate, the cholesterol synthesis is blocked, low density lipoprotein decreased the blood total cholesterol decreased by 25% to 35%, low density lipoprotein decreased from 30% to 40%, thereby reducing the blood lipid level. There is clinical evidence that statins have important clinical significance in the prevention of coronary heart disease, stroke and treatment of acute stroke.
Statin market CR4 was 81.9%, CR8 was, belonging to the high oligopoly market. The data show that the growth of statins mainly comes from two kinds of atorvastatin and rosuvastatin.
The film as the representative of Pfizer's Lipitor atorvastatin brand advantage, build a high barriers to market intervention, market share is as high as 50% (see Table 1). Lipitor for the first time in 1996 approved the listing, in 2004, to become the world's first Lipitor sales exceeded tens of billions of dollars in drugs. In 2007, the FDA approved atorvastatin for non fatal myocardial infarction, stroke, cardiac surgery, heart failure and heart disease, chest pain and 5 additional indications, so as to consolidate its leading position in the treatment of hyperlipemia drugs in the market, its remarkable curative effect and safety of Lipitor driven the rapid growth of market share. In November 30, 2011, the Lipitor patent protection in the United States due, one after another in China there are many enterprises to imitate, in recent years the market share has declined, but the market is still difficult to shake the status of lipitor.
In recent years, the market has expanded rapidly, although the brand is not much, but in the list of two brands of statins has been the seat. The AstraZeneca tablets ranked second, Shu tablets (Nanjing Xianshengdongyuan) ms shaftan ranked fifth. May 2012 to the first half of 2016 sales of tablets of the semi annual compound growth rate of 38%, Shu Grafton tablets compound growth rate of three digit growth, big growth and Nanjing cttq support properly, has not yet entered the top ten, but the performance is still more prominent.
 Statins not only through the lipid-lowering effect of a single, but also through the stability of plaque, inhibit inflammation, improve endothelial function, inhibition of platelet aggregation, such as the synergistic effect of multi way to complete lipid-lowering. They are the first-line drugs for the prevention and treatment of dyslipidemia, which has been widely used in our country. Can be predicted, in China's pharmaceutical retail market, statins as a regulator of lipid drug market in mature varieties, the future will continue to dominate the lipid regulation drug market.
Heavy drug Lipitor and Crestor
How many cows
Lipitor, the Pfizer Inc original products, approved by the FDA listed in 1996, approved at the beginning of may even when the researchers do not have anticipated, will become the first global prescription drug lipitor. 2006 global sales of Lipitor peak of $12 billion 900 million in 2007, although sales started declined slightly, but from 2004 to 2010 for 7 consecutive years, Lipitor sales of more than $10 billion, have to say is a miracle. In the history of medicine, it is also the first to break the billion dollar mark of the heavy drug king. Although from the beginning of November 2011, Lipitor have lost patent protection in the United States and other places. But it is still a trump card in the global retail market, especially in developing countries.
Can be set, and Famory rosuvastatin, 2003 approved by FDA, is after Lipitor |
