Medical network on March 15 - recently released the 2017 edition of national medical insurance directory compared with the 2009 version of the directory 339 new varieties, including western medicine increase 62 exclusive varieties. In the new list of exclusive varieties, domestic medicine companies involved in more than 20, hengrui medicine, beida pharmaceutical and shiyao, method and so on among both in research and development ability of enterprise. Foreign drug companies, novartis has six exclusive varieties to enter. Followed by glaxosmithkline, there are three exclusive varieties to enter. In addition, Pfizer, boehringer ingelheim, daiichi sankyo, also each have two exclusive species into new medical insurance directory.
Concern of domestic enterprise key exclusive varieties are: shiyao main varieties will's injection, beida pharmaceutical for ek, and method of moxa oprah, simcere ella maud, hengrui iresearch yesterday.
Forecast according to professional personage, for new medical insurance directory varieties, sales this year is expected to impact is not big, next year is expected to moderate, and the final realization of sales income rapid growth depends on its clinical value.
The current domestic enterprise innovation products have entered the harvest, the next few years and see who will become the new "dark horse"?
Shiyao grace will: high growth for six years
Butyl phthalide soft capsule by shiyao group listed approved production in 2005, commodity called "will", mainly is suitable for the treatment of ischemic stroke (commonly known as stroke). In April 2010, butyl phthalide and sodium chloride injection approved production, the success of the new dosage form listed add vitality to the market. At present the product has become a shiyao group fist products.
Grace will stroke is widely recommended in clinical medicine, mainly used in the treatment of mild and moderate acute ischemic stroke. The drug is artemisinin, bicyclol after third world recognised China's original new chemical entity drugs, also is the only global with mitochondrial protection of cerebral microcirculation reconfiguration agent, there are butyl phthalide soft capsule and butyl phthalide and sodium chloride injection two dosage forms. In 2010, shiyao group "butyl phthalide raw materials and soft capsule" project won the national scientific and technological progress second prize.
Samples according to the domestic hospital statistics, in 2005-2009, butyl phthalide market has been in the doldrums, butyl phthalide samples of domestic hospital drug use amount maintained in 2 million - 6 million yuan between, for the past five years have not exceeding RMB ten million. In 2010-2016, grace will market a rapid rise, present high growth momentum for six years, from 1.59 million yuan in 2005 sales growth is expected in 2016 to 2016 yuan, of which, butyl phthalide capsule sales of 440 million yuan, butyl phthalide injection sales of 240 million yuan.
In 2010, butyl phthalide oral often release dosage forms into the 2009 national health insurance directory and will be successfully listed at injection dosage forms, become a turning point in the market, the rapid growth of product sales. In 2017, butyl phthalide injection after entering the new medical insurance directory, will be at the market is expected to speed up.
Baida kaimei: seize market at a staggering rate
Ek for from beida pharmaceutical development, belong to EGFR tyrosine kinase inhibitors, first-line treatment for non-small cell lung cancer. In June 2011 by CFDA approved patent expires in 2023 (compounds), trade name "kay beauty". Formulations for the tablet, the specification is 125 mg. Space is big, small molecules targeting lung cancer drug market in China for ek's growth potential.
In domestic for driven type of clinical medicine, medicine for ek, domestic exclusive innovation gradually broke the imported drugs monopoly market situation and market rapid rise for his class. In 2016, ek chemical pharmaceutical industry in our country for Nepal's first "national science and technology progress award", this is the first since the founding of the people to get the award of the domestic chemical synthetic drug.
In May 2016, the state development planning commission announced tenofovir, ek countries the treatment for and price negotiation as a result, the negotiation result in 2016-2017, and take steps to make procurement, medical insurance policy and price negotiation results. In 2017, tenofovir, ek for the treatment and three products have been included in the new national health insurance directory.
According to the domestic sample hospital statistics, from 2011-2016 is expected to sales trends, ek for 4 years in a row to present high growth momentum. 2011 annual sales of 4.84 million yuan. 2014 annual sales of 150 million yuan. 2015 annual sales of 210 million yuan, a 36.2% increase from the same period. Expected 2016 sales of 240 million yuan, a 14.4% increase from the same period. In recent five years, at a breathtaking pace, seize more market share for ek, has become a domestic small molecular targeted drugs market a new bright spot, but in 2016 is expected to market has slowed.
Beida for ek, undoubtedly has a pivotal position in the Chinese new drugs, the drug was hailed as "atomic" one of the most important innovation of biomedicine, and has been regarded as a domestic "Yi Rui sand". As the product into the new health insurance directory, believe this will have a new for ek, in the market.
Method one lian: series of good to speed up the layout
Ai oprah azole is a potent ion pump inhibitor, in December 2007, approved by method group, commodity name one lian, dosage form for enteric-coated metformin hydrochloride, specifications for 5 mg. The product to be group of independent innovation of new drugs, has been applied for, the United States and the European Union, Australia, Japan, and a number of countries such as invention patent, become the company's new profit growth point method. In January 2016, the product won the 2015 national scientific and technological progress second prize, as the blockbuster products in the field of the digestive tract, nearly five years a hat-trick of market.
Domestic listed six proton pump inhibitors, respectively is orchid sola, panxi tora etc, omeprazole, esso beauty pull azole, rabeprazole azole and ai oprah, including moxa oprah azole listed at the latest, and is the sole product.
Hospital according to the sample data statistics, in 2008 Mr Azole sales of 870000 yuan, 2015 sales of 2015 yuan, up 42.9% from a year earlier, rapid growth, is expected to 2016 annual sales of 120 million yuan, a year-on-year increase of 36.1%. In recent years, Mr. Pula azole market explosive growth, growth to be reckoned with.
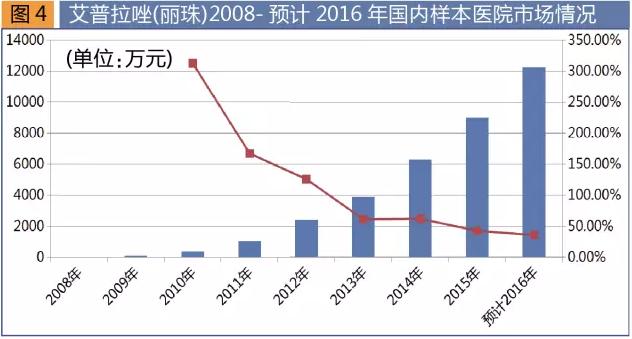
We have more kinds of the current treatment of peptic ulcer drugs, among them, the proton pump inhibitors is currently the strongest acid suppression effect, clinical curative effect is the best type of drugs. Notable is that the ai oprah azole sodium injection has been included in the list for priority review procedures of drug registration, as Mr. Pula azole enteric-coated metformin hydrochloride to join the new health insurance directory, coupled with the subsequent innovation medicine moxa oprah layout of new dosage form, will bring a new round of growth for the company, at the same time will also boost the rapid growth of the gastric acid secretion drug market in China.
First sound Aitken singh: growth in nearly three years
Ella maud is obtained by simcere in 2011 listed on the world's first 1.1 class new medicine, belongs to rheumatoid arthritis drugs, called "ai xin" goods. Ai sheen's successful listing is important milestone in China's science and technology research work for prevention and treatment of rheumatic diseases, marked the our country in the field of anti-rheumatism medicine development has become a world leader.
Simcere production Aitken sheen on sale began in 2012. As a kind of drugs alleviate, ai have to work quickly, better efficacy and safety, with a full range of bone protection. Sample according to the domestic hospital statistics, for four consecutive years 2012-2016, the products present high growth momentum. Sales of 2.61 million yuan in 2012, 2014 annual sales of 16.01 million yuan, 2015 annual sales of 26.94 million yuan, a 68.2% increase from the same period, is expected to 2016 annual sales of 35.8 million yuan, a 32.9% increase from the same period. Ella moder of relatively slow growth in 2012-2013, 2014-2016, three years is expected to rise faster, the market performance is more outstanding.
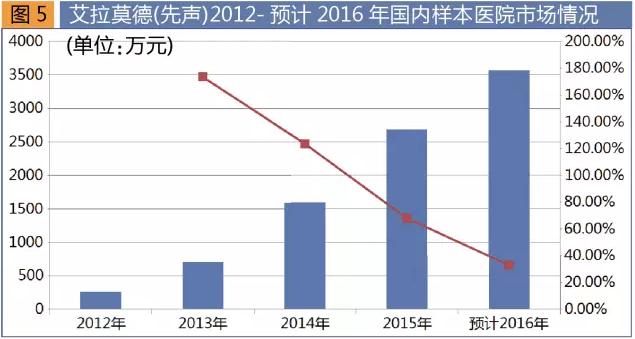
Treatment of rheumatoid, on the international at present main products are used in the treatment of rheumatoid arthritis symptoms, no small molecule drugs for the treatment of autoimmune diseases, and ella moder listed really fills the blank. After the products onto the market two years ago, due to not catch up with the national health insurance directory of adjustment cycle, the situation has been difficult to quickly open the market, sales volume is limited by a lot. The ella moder after entering the new medical insurance directory, with the support of health policy, is worth looking forward to the future market.
Enviable hengrui heng Yang: rocket speed
Iresearch yesterday cloth is independently by jiangsu hengrui pharmaceutical research and development of new drugs, in May 2011, commodity called "heng Yang", dosage forms of tablets, the first in the world. Compared with traditional medicine, higher safety and better compliance, iresearch smoothie cloth is first-line therapy for joint pain, osteoarthritis. The product in reducing bone arthritis symptoms of pain and gastrointestinal safety and the effects of celecoxib, cardiovascular, renal function, better safety, the drug post-marketing Pfizer as celecoxib's main rival.
Hospital according to the sample data statistics, iresearch yesterday cloth the sum of 1.03 million yuan in 2012, 2014 to 6.99 million yuan, in 2015 to 2015 yuan, up 56.7% from a year earlier, rapid growth, is expected to 2016 annual sales of 21.17 million yuan, a year-on-year increase of 93.3%. 2013-2016 is expected to sales growth of 105.1%, 56.7% and 93.3% respectively, the high speed growth for three consecutive years.
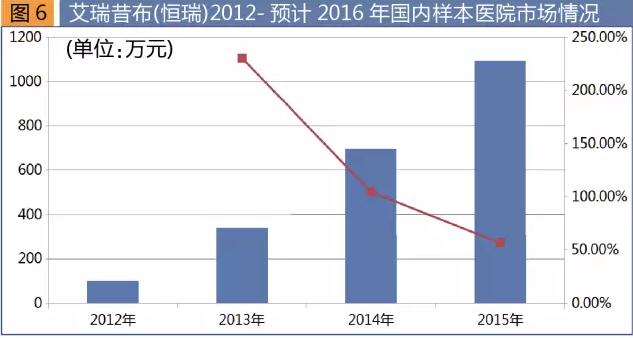
At present, with the aging of the population, the incidence of chronic diseases increased year by year, the joint drugs market is a huge market. Iresearch yesterday cloth is China's first ever kind of independent brands, hengrui with product advantages and huge marketing network, combined with the advancement of the new medical insurance directory, in the next few years product sales will gradually. Innovative drugs to harvest hengrui driven growth performance has played a more obvious effect, after entering the new medical insurance directory will accelerate the import substitution, the future prospects. |
