Medical network on March 21 - nonalcoholic fatty liver disease (NAFLD) as the western developed countries the most common liver disease. Launch in April 2016, the European society for liver disease in the European Union's first "nonalcoholic fatty liver disease (NAFLD) guidelines. The guidelines defined NAFLD liver fat excessive accumulation associated with insulin resistance, characterized by liver biopsy fatty change affects more than 5% of the liver cells or 1 h - magnetic resonance mass spectrometry (1 h MRS) changes of the liver cells.
With the rapid development of China's modernization, fatty liver has become China's important liver disease. General office of the national health and family planning commission issued on December 2, 2016 by the nonalcoholic fatty liver disease clinical pathway (2016 edition) ", further improve the effect of clinical pathway management and implementation. Its aim is to enhance the understanding of the patients with NAFLD, raise the level of human health. At present, nonalcoholic fatty liver disease drug market performance?
Fatty liver billion-dollar dinner to be reckoned with
Nonalcoholic fatty liver disease (NAFLD) incidence in the western developed countries occupy 20% ~ 30% of adults, including 10 ~ 20% of the patients with nonalcoholic fatty liver disease can progress to nonalcoholic fatty hepatitis (NASH). In recent years, the global incidence of nonalcoholic fatty hepatitis is 3% - 5%. Predict 2030 years, nonalcoholic fat hepatitis may exceed the hepatitis b virus, become a serious threat to human health problems.
Related report shows that our country community adult prevalence of NAFLD fluctuations in 10% ~ 10%, which is similar to the epidemiological data of North America roughly. While under the influence of age, gender, race and genes, as the life rich, excess intake of energy and visceral obesity, type 2 diabetes and the metabolic syndrome and the effect of liver histology changes including simple fatty liver, non-alcoholic fatty hepatitis to cirrhosis and liver cancer of the disease. Therefore, nonalcoholic fatty liver disease not to be sneezed at.
Currently, the FDA has not yet been formally approved for nonalcoholic fatty liver disease treatment, the clinical application of nonalcoholic fatty hepatitis drug is still rare. For the treatment of fatty liver disease, mainly protect liver to protect liver drugs combination therapy, its huge market outlook has become a consensus. According to research firm, according to the 2015 global nonalcoholic fatty liver disease treatment the size of the market has reached more than $10 billion. Notable NASH is in the research pipeline drugs are not much, but there have been many pharmaceutical companies compete for treatment of nonalcoholic fatty liver disease.
Fatty liver treatment market structure in China
Liver disease belongs to the independent discipline, more domestic drug for the treatment of liver disease, mainly by the patient poison resistance, protect liver to protect liver and fatty liver disease resistance of drugs and treatment of hepatic coma. In 2015, according to according to the data published in kang CMH domestic urban public hospitals, public hospitals at the county level, the urban community health care, in towns and townships, physical pharmacy, store six big terminal liver disease drug overall size has reached 44.03 billion yuan, the growth rate of 8.3%.
Auxiliary liver disease treatment on cell membrane structure and cell metabolism has a good stability, can resist the liver cell necrosis, relieve fatty degeneration, promote protein synthesis, inhibit the rise of alanine aminotransferase, through the cholagogic, detoxification, inhibit the generation of free radicals and lipid peroxide formation, to repair the damage of liver cells, protect the liver.
People on the improvement of liver disease recognition and emphasis, and promote the rapid development of a series of liver disease drug, has been formed by chemical medicines, plant extracts are engaged in three series of traditional medicine and market pattern. Among them, protect liver to protect liver and fatty liver drug resistance present a fairly obvious sustained growth momentum.
In recent years, the rapid growth of the market in the new drug research and development of our country situation, the combination therapy of liver disease and auxiliary treatment market has a comprehensive change, the product structure is mainly composed of the drug that protect liver, fall enzyme drugs, jaundice, liver fibrosis, alleviate inflammation drugs of five types. Liver disease drug treatment has gradually become the administration with large amounts of money.
According to the PDB data statistics show that in 2016 major cities in our country public hospital liver protective drugs have 50 of generic drugs, drug use amount is 3.958 billion yuan, year-on-year growth of 9.19% a year, about 60% of the key city public hospital liver disease drug market. In 2017, the national health care medicine catalogue contains liver medicine treatment, resisting fatty liver 14 varieties of western medicine, are into the liver disease of adjuvant TOP30 varieties.
At present, the fatty liver medicine varieties need medicines respectively, glutathione, glycyrrhizic acid magnesium, compound glycyrrhizin glucoside, polyene phosphatidyl choline, nmda ornithine, adenosine methionine, dichloroacetic acid diisopropylamine, glycyrrhizic acid, ammonium, methionine fetal liver extract, vitamin B1, compound cattle these are hospitals awareness higher drug that protect liver.
Glutathione development made new progress
Glutathione is formed by the junction of glutamic acid, cysteine and glycine into three substance p. At present, has developed the glutathione drugs, can be used to promote the metabolism of sugar, fat and protein in the body, accelerate the elimination of the free radicals in the body, and protect the liver synthesis, detoxification, inactivate hormone, and other functions, is mainly used for clinical treatment of liver disease and alcoholic liver, fatty liver, the poisoning and the adjuvant treatment of viral hepatitis or other better.
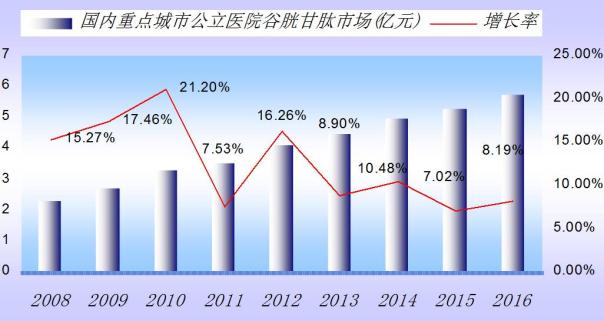
Reduced glutathione is the human nature of a kind of peptide synthesis in the cytoplasm, composed of glutamic acid, cysteine and glycine. In 2016, the domestic main city public hospital glutathione is fatty liver on top of drug resistance, has reached 573 million yuan, year-on-year growth of 8.19% a year. CFDA approval 5 domestic production of glutathione oral and injection preparation, in addition to the imported drugs. Hospitals use glutathione TOP5 drug is chongqing medicine friends tinto o moran accounted for 45.91%, Shanghai fudan after China pharmaceutical the double benefit of national health account for 20.33%, Italy, the pharmaceutical industry gura occupy 17.88%, of loose the large kunming, account for 8.34%, shandong green leafy green dean to account for 6.3%. That domestic glutathione the size of the overall market has exceeded 5 billion yuan, with the market demand, will show a steady growth trend.
Glycyrrhizic acid series of drug fast growth
Since the 1950 s, the plant medicine active ingredients of licorice glycyrrhizic acid were discovered, the study of the mechanism of drug action in the further. Of glycyrrhizic acid has anti-inflammatory, antioxidant, anti liver poison, the stability of cell membrane, liver fibrosis, immune regulation, inhibiting the Ca2 + internal flow, prevent apoptosis, as well as various effects such as increased endogenous steroids produce to have comprehensive understanding, thus promoting the glycyrrhizic acid series of drugs in the clinical application of liver disease clinically applicable to the ductility of chronic hepatitis, chronic active hepatitis, acute or chronic hepatitis b, liver poisoning, resisting fatty liver and early cirrhosis of the liver.
Glycyrrhizic acid series of drugs is nearly five years, the rapid growth of the categories of drugs glycyrrhizic acid drugs registration approved by the state approval, more than 280 copies. With the broadening of clinic application, promoting the rapid development of glycyrrhizic acid preparation, licorice mixture, now from the original extract, the first generation of glycyrrhizic acid preparation glycyrrhizin tablet and the second generation of glycyrrhizic acid in beta body single ammonium salt as the main ingredients of compound glycyrrhizin glucoside preparations, developed the third generation of glycyrrhizic acid preparation of alpha and beta body blending glycyrrhizic acid ammonium salt and the fourth generation of glycyrrhizic acid preparation glycyrrhizic acid magnesium, gradually formed in the oral interpretation of tablet, capsule, injection and booster injection preparations four big dosage forms of the new pattern of glycyrrhizic acid series varieties.
In 2016, the domestic major cities public hospital glycyrrhizic acid series of drug use amount is 1.198 billion yuan, year-on-year growth of 13.13% a year. Mainly different glycyrrhizic acid magnesium, compound glycyrrhizin glucoside, glycyrrhizic acid, ammonium, compound glycyrrhizin, compound glycyrrhizin, glycyrrhizic acid, ammonium single single potassium, glycyrrhizic acid, ammonium cysteine, etc. 8 varieties.
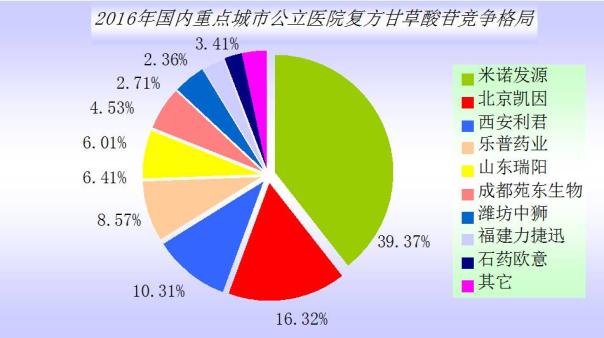
In glycyrrhizic acid series of drugs, pharmaceutical jiangsu zhengda shine the exclusive varieties of different glycyrrhizic acid magnesium injection luscious (trade name shine) is the sample hospitals of glycyrrhizic acid drug use amount of the first series varieties. Different glycyrrhizic acid magnesium is zhengda shine to adopt advanced technology of chiral successfully developed the global the first of glycyrrhizic acid containing alpha glycyrrhizic acid with high purity magnesium products, won the state intellectual property office of the new compound invention patent authorization and the tenth China patent prize award.
In 2016, the domestic major cities public hospital luscious market is 550 million yuan, the weather is fine year-on-year growth of 12.41% a year. Joint glutathione treatment of fatty liver have good curative effect, is to improve the abnormal liver function and liver cell protective agent.
Glycyrrhizic acid series drugs sales second is compound glycyrrhizin glucoside, is to protect the liver cells, nonalcoholic fatty liver disease resistance, resistance to drugs of liver fibrosis, glycyrrhizin, glycine and methionine compound, dosage form is oral preparations and injections. Mino CFDA approve the import drug is Japan origin of pharmaceutical drugs can "beauty", approval of 19 domestic enterprises producing compound glycyrrhizin glucoside.
In 2016, the domestic key city public hospital compound glycyrrhizin glucoside market for 380 million yuan, year-on-year growth of 4.28% a year. Top 10 pattern to show the beauty import drugs can occupy 39.37%, Chinese medicine account for 60.63%.
Nmda ornithine market rebound
Nmda ornithine is used in the treatment of fatty liver drugs in glutathione, glycyrrhizic acid in third place after a series of drug varieties, suitable for various hepatitis, fatty liver, liver cirrhosis and hepatitis syndrome after treatment. Nmda ornithine and glutamine can provide urea synthetic substrates, detoxification in liver cells, promote the liver cell energy generated at the same time, the injury of liver cell is the function recover rapidly. Our country has imported German Merz Pharmaceuticals GmbH nmda ornithine injection, held trade name yobo division (Hepa Merz); 2006 Wu Hanqi rui pharmaceutical nmda ornithine granules and powder injection of generic, brand name red, still the domestic nmda ornithine agent exclusive varieties; 2011 CFDA chongqing mastery in pharmaceutical industry (group) the approved Po SAN pharmaceutical nmda ornithine API approval.
In 2016, the domestic key city public hospital nmda ornithine market is 281 million yuan, year-on-year growth of 4.85% a year. Including Wu Hanqi pharmaceutical industry occupies 90.96% market share, Germany Merz accounted for 9.04%.
Fatty liver and liver auxiliary medicine market prospect
As people in liver disease antiviral differentiation to the comprehensive understanding of drug use and auxiliary treatment. Samples in the past two years, the hospital drug use rankings are also constantly refreshed, from a variety of mechanism combination has become a trend for the treatment of fatty liver. And the top 20 or more drugs in compound preparations and proprietary Chinese medicine, and this is because the patients to the side effects of small compound the favour of proprietary Chinese medicine preparations, promoted the compound proprietary Chinese medicine preparations. In the medicine, dichloroacetic acid diisopropylamine, compound bovine liver extract tablets, methionine, vitamin B1, beauty he xin, Mr Lamy, six dimensions lecithin soft capsule and so on also has a good market, is the domestic hospital channel high-profile category. |
