Medical network - on February 23, the state council general office on February 9, issued "on further reform and improve the use policy for producing and distributing medicines of several opinions" (hereinafter referred to as "certain opinions"), policy, released from the Angle of the whole industrial chain upstream and downstream compliance of guiding significance to clear the pharmaceutical industry. Under the strict control of production circulation use link, profit is the basic pattern of distribution, and small profit era officially.
Pharmaceutical industry once owned system of demographic dividend, bonus, WTO dividends, bonuses, resources and productivity bonuses, financial and trade dividend growth is positive, such as under the medical insurance fund pressure, these bonuses has been basically to the end.
Science and technology and information technology dividend will be the future growth of our country's pharmaceutical industry. However, under the background of information flat, the enterprise competition, China's pharmaceutical industry reshuffle is still in the short term, long-term positive left over after the "fittest" enterprise.
[small profit engine]
Price transparency, the profit allocation decision project preferences
"Certain opinions" referred to in the drug prices have (port) drugs leave the factory price, the drug bidding procurement price, medicine health care given price, payment/settlement price, etc. As "two votes" aside, the food and drug supervision, health, family planning, human resources, social security, price, taxation, business administration, public security departments to crack down on the price fraud and price-fixing violation behavior, combined with the drugs price monitoring system sound, drugs ex-factory price information, the establishment of the traceability mechanism as well as to the country of origin or the surrounding collection "combination", such as price, price of each section of drug information basic transparency. The tax characteristics of VAT also let profit information is more and more transparent.
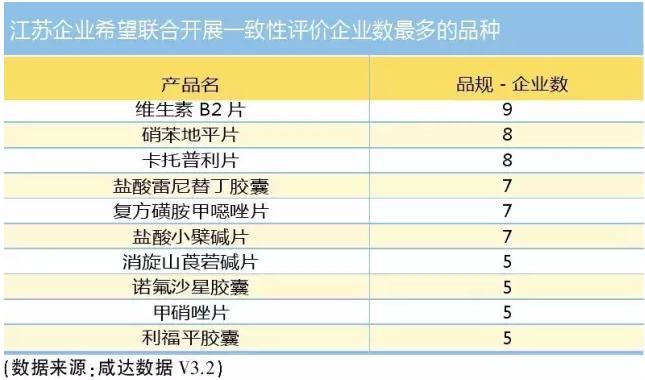
Under basic determine price and profit, encourage enterprises to choose the product market profits nature project will pay more attention to the potential market. The research and development costs and fees, the success rate of project and risk information transparency as well. Suppose that a generic project before approval documents for production enterprises of investment is 10 million yuan, the future competitive manufacturer of 5 ~ 10 home, considering the market investment and subsequent brand pharmaceutical production cycle, the manufacturer will tend to choose $terminal sales potential projects. For generic drug sales is bigger but the price is lower, more companies will choose "unity", the enterprises hope to jointly released from jiangsu consistency evaluation table varieties can also see that sales of the larger vitamin B2, nifedipine pills and medicine such as captopril tablets is product enterprise more hopes of cliques.
Active ingredients in compound/statistics, 2015 samples of hospital one hundred million yuan of the size of the market products a total of 342, the 342 products includes face shrinking in under the tide of the rational use of drugs and dosage of auxiliary medicine and nutrition drugs and other products. "Certain opinions" will push the sales terminal dosage information more transparent, this means that on the one hand, the dosage of the auxiliary medicine and nutrition drug products such as will be strict limits on future listing is likely to play one hundred million yuan; List of products on the other hand, one hundred million yuan is the variety of the generic drug manufacturers will contend for, will be more competitive.
The registration information will be found that most of the $project has been zhengda shine, hengrui, hogan &hartson, qilu, nolato, not before unleashing enterprises in research and development projects such as "buy buy" layout in advance, small and medium-sized enterprises want to play now to participate in generic competition, will take the cost of 5 ~ 10 times.
On funding of small and medium-sized enterprises, which leads to the first year most into three projects. Small and medium-sized enterprises to choose three direction of the project:
One is choose according to their own enterprise sales strong field sales lead, the competition of the larger project consistency evaluation of the location of the top five or even in the first three.
2 it is to avoid big companies choose hot area, choose less competition and product project cycle relatively longer segment small areas, such as rare diseases.
Three is to choose the project of no clinical, the cost is low, preparation aspect such as injection, eye drops, therapeutic areas such as child medicine and traditional Chinese medicine (TCM) by the party, etc. It is important to note that these products are not necessarily large enterprises not layout, eight hengrui is eye drops production approval, including ratan other eye drops, maleic acid thiamethoxam bisoprolol eye drops and maintain eye drops of hydrochloric acid, hydrochloric acid hydroxyl methyl thiazole moiety eye drops, naphthalene min d eye drops, tobramycin eye drops and tobramycin dexamethasone eye drops seven of generic products. During the reporting/approval documents have clinical products three, respectively is pat eye drops, benzene sulfonic sting his eye drops and the other eye drops.
[pattern rearrangement]
"Product is king" and "channel is king" new bureau
Started to fault line
Production enterprises in the short or medium term state "of the pharmaceutical industry concentration" policy under strict controls of increasing pressure, facing short-term policies are mostly about "raw material change, process adjustment" production inspection, long-term facing the market pressure involved in clinical pathway management policy to the ground, a medical representative academic promotion behavior of severely limits on the rational use of drugs, such as individual products still faces "two votes" separate "medical" channel market after compression, the above policy will promote the products market structure changes.
New demand for new drugs, generic drugs need consistency evaluation, clinical trial data check strictly, the threshold of the domestic drug approval is approved in ascension. "Certain opinions" advance, listed products in China is expected to common name, the number of drug approval, manufacturers as a whole will continue to decrease, the auxiliary medicine, nutrition, medicine etc approval will be short-term impact is bigger. Mass production manufacturer in the hands of product approval to be invalid, the new approval took less than, fault line, was forced to withdraw from the market. Finally reached "push backward enterprises exit, strive to dissolve the pharmaceutical production enterprise quantity, small scale, low level problem" goal.
In "certain opinions" short-term positive has layout product line ahead of schedule and project quality of enterprises, especially in the United States has been successfully listed in generic drug products, such as hengrui, huahai, nolato, and China resources, etc.
Product structure adjustment, auxiliary medicine and nutrition to the main income for antibiotics drugs production enterprise and the downstream distributors. In the short term this production enterprise survival pressure, the biggest desire to seek transformation is the most urgent. Project cost determines the enterprise project was not as simple as in the past repeatedly, the role of information retrieval, information integration in project more and more important.
Project cost will lead to high sales limited low medicine has the power to carry out consistency evaluation project, such products in the future may be more state-owned enterprises designated production, supply of fixed-point.
New game oligarch competition reversed transmission channels
The implementation of the "two votes" in addition to boost circulation industry concentration increase, can also lead to industry of current levels of flattening. Separate "medicine" is more of a channel diversion work, social pharmacy share the original hospital outpatient pharmacy to work. "Certain opinions" to encourage the drug circulation downstream integration, thus forming the drug circulation enterprise integration of wholesale and retail business, this means that the terminal in our country in the future competition pattern is mainly several large circulation enterprises in the integration of large chain retail pharmacy after the "big MAC" enterprise (e.g., China resources, national medicine, medicine) between the oligarch competition situation.
Clinical medical institutions, hospital clinical application directory if reference for anhui, fujian, from the first clinical demand determine various disciplines required medication directory into another province specialized hospital procurement directory, and with the development of clinical pathway basic cure the hospital drug use directories, eventually the national medical institutions clinical drug directory structure coincidence rate is expected to be as high as 70% above, not like now, the gap between the larger hospital clinical application directory. Market access to medicines for medical institutions and future of basically is the clinical evidence.
The change process of circulation industry is also the process of China's pharmaceutical industry and drug structure change. Pharmaceutical enterprises and the circulation use link under the condition of strong, short-term production and circulation among two big head will be asked to link profit squeeze, agent, pharmaceutical representatives and doctor prescription commission will face cuts. In the long term, large commercial firms will be starting from the product development cycle, control access to generic drug manufacturers guarantee of profits, small and medium-sized generic drug production enterprises tend to choose a big form close cooperative relations to ensure the commercial monopoly sales channels covering.
Extension > > >
Think of the future dividends
Social media recommended drug use, electronic payment in universal coverage of medical institutions, etc., science and technology and information technology dividend will be the future growth of our country's pharmaceutical industry.
But how information technology will reverse the effects of the pharmaceutical industry has yet to reflect for the time being. The national development and reform commission in 2016 edition of the catalog for the guidance of strategic emerging industries focus on products and services mentioned "data pharmaceutical" "artificial intelligence system" is not reflected in the "several opinions".
In addition, the marketing authorisation holder system in the short term is mainly used in foundry production, for r&d incentive effects of long-term observation is needed. The long-term incentive system of marketing authorisation holder pharmaceutical industry division of labor refinement, if the country's major drug discovery science and technology major projects such as the national science and technology plan (special, funds, etc.) supporting in place, with tiny scientific research institution of frontier science and technology dominated by scientists will rise. |
